Carbon monoxide poisoning - What you need to know
Last week, the country experienced the tragedy of a mother and her two children in St Mary dying from carbon monoxide poisoning. Carbon monoxide poisoning rarely happens by accident in a warm country like Jamaica, where homes and other buildings are open to external air circulation.
But each year, several cases occur in cold countries where buildings are closed off from free air circulation, especially in winter for retaining heat. People have also died in their motor vehicles when snow has blocked the exhaust pipe, pushing exhaust gases, which include carbon monoxide, into the cabin of the vehicles. This happened in the recent massive snowstorm in the Northeast United States.
Carbon monoxide - a colourless, odourless, tasteless gas - is produced when fuel and other carbon-containing organic materials are burned. The St Mary family had been operating an electrical generator inside their house during a power outage.
Safety regulations require that generators are operated in open spaces with free air circulation precisely for this reason.
PROCESS OF OXIDISATION
When carbon-containing material like gasolene burns, the carbon content combines with oxygen in the air to form oxides of carbon. When sufficient oxygen is available, the end product is carbon dioxide, an oxide with two oxygen atoms attached to a single carbon atom. In insufficient oxygen, only one oxygen atom is attached to the carbon atom, so carbon monoxide.
Our own bodies, like other living organisms, produce non-toxic carbon dioxide from the 'burning' of food fuel inside our cells which is called respiration and we exhale this CO2 through our lungs.
What makes carbon monoxide, CO, a deadly toxic substance is what is does to blood. Haemoglobin, the stuff which makes blood red, carries oxygen from our lungs to every cell in our bodies. The oxygen hitches on to the haemoglobin in the lungs where there is plenty of oxygen, but lets go quite easily in the tissues where there is little oxygen. This is like tying on the oxygen molecule to the haemoglobin with a weak string, which is easily broken.
Carbon monoxide also hitches on to haemoglobin, but with 230 times the strength of the oxygen tie. That is 230 wraps of the string! And CO does not let go. So the haemoglobin in blood is not available for binding and transporting oxygen.
The victim of carbon monoxide poisoning quite literally dies of suffocation in the midst of an abundance of oxygen which can't be taken up.
Treatment for victims who are still alive is basically by hyper-oxygenation, getting the patient to breathe a mixture with a high concentration of oxygen and which may be pressurised.
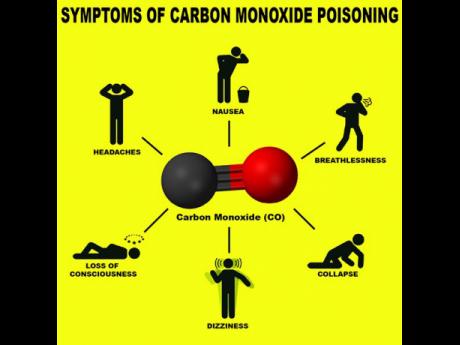
When people are awake, carbon monoxide poisoning initially induces headache, nausea, malaise and fatigue. The malaise, and fatigue are particularly dangerous, as people lose the drive and the mental coherence to take corrective action. When people are asleep, they simply never awaken.
In our situation, avoiding toxic levels of carbon monoxide, which are levels greater than 100 parts per million, is easy: Never burn fuel or operate fuel-burning machinery in closed spaces that do not have free air circulation.
The rare St Mary carbon monoxide poisoning tragedy need not happen again.
Email: yourhealth@gleanerjm.com